Medical professionals, driven by the influence of pharmaceutical marketing and decades of biased media coverage, frequently exaggerate the benefits and safety of selective serotonin reuptake inhibitors (SSRIs), popularly referred to as "antidepressants." This inflation of their efficacy perpetuates a skewed understanding of these drugs, while crucial safety concerns remain underreported and underestimated. As a result, patients are deprived of the necessary information to make truly informed choices about their mental health treatment.
As the use of prescription antidepressant drugs continues to increase, there has been a growing and vocal global community of patients who have experienced harm. This situation is further complicated by the fact that at least 80% of these drugs are prescribed by under-qualified medical professionals in primary care settings. These healthcare providers often lack the necessary knowledge to accurately assess and treat mental health problems.
The time constraints of an average visit, typically less than 15 minutes, pose significant challenges in developing a genuine understanding of a patient's emotional struggles. This limited timeframe makes it exceedingly difficult for healthcare professionals to thoroughly evaluate a patient's condition and make informed treatment decisions. Consequently, this alarming trend leads to a hazardous scenario where mood and mind-altering medications are excessively prescribed for normal reactions to stressful events.
Off-label prescribing of antidepressants is now common for a myriad of health related conditions. Even in cases where someone is genuinely suffering with a prolonged depressive episode or severe anxiety, the risks of SSRI use are rarely provided in a meaningful way.
Given the current controversy on SSRI safety and efficacy patients must evaluate the potential for harm when consenting to a prescription drug, especially when safer or more effective interventions exist. It becomes even more complicated for children, teens & young adults where the dangers may even be greater. I will provide a more thorough review for children and adolescents in future posts.
What is Informed Consent?
Informed consent is a crucial aspect of medical practice and ethical decision-making. It ensures that patients have the necessary information to make informed decisions about their treatment options and care. In the United States, the requirements for informed consent vary depending on state laws and regulations, as well as the type of medical procedure or treatment being offered.
Generally, informed consent involves the patient's understanding of the risks and benefits of the proposed treatment or procedure, alternatives to the treatment, and the patient's right to refuse or withdraw from treatment. In cases where the patient is unable to provide consent due to impairment or incapacity, informed consent may be obtained from a legal guardian or surrogate decision-maker.
What Have the Clinical Trials Revealed?
After thoroughly examining the vast body of scientific literature, it is difficult to conclusively state that antidepressant drugs are superior to placebo. There should be stringent criteria to establish the safety and efficacy of these medications. The FDA approval process is designed to get drugs to market as quickly as possible and the clinical trials are developed to meet these FDA guidelines.
In a much-debated paper published in 2002, using the Freedom of Information Act, Irving Kirsch obtained trial results for all SSRIs approved between 1987 and 1999. Kirsch showed that the drugs lowered patients' scores on the widely-used 52-point Hamilton Rating Scale of Depression only 1.8 points more than did placeboes, a difference that's statistically significant yet almost meaningless. The results from his analysis have been replicated and the debate on whether antidepressants provide any real world clinical improvement continues today.
When analyzing a collection of published trials, as in this meta-analysis paper, it is clear that antidepressants have little advantage over a placebo - an inactive sugar pill. Many people reading the papers do not possess the training to interpret the data and rely only upon authors conclusions. Most guidelines (which many clinicians rely on to guide treatment decisions) do not fully acknowledge the poor quality of the data on which they are based.
A statistical significant difference does not mean the drug is clinically useful, or even that the drug works as proclaimed. The difference between the two is so small that it raises questions about the accuracy of the studies. There are various methodological issues with these trials that may account for the minimal variance between the drugs and the placebo. People who take the placebo appear do just as well. Therefore, it is difficult to determine whether there is indeed a discernible difference between the two, or whether the results are due to other factors.
Physicians and patients often assume that the drug trials were designed with high rigor and that the long-term safety of the drug has been established. However, most trials were only 6-8 weeks in length and pharmaceutical companies can manipulate trial results to create a statistical difference between the drug and placebo groups. This can include excluding suicidal clients from the trial and removing patients who became suicidal after being given the drug from the final data analysis. In addition, placebo group patients may be abruptly taken off their current psychiatric medication, inducing withdrawal symptoms, to enhance the perceived efficacy of the drug being tested. As a result, the trial data may not reflect the true efficacy or safety of the drug in real-world settings.
How Did These Drugs Get Approved?
Pharmaceutical companies have the ability to conduct numerous trials until they obtain the two statistically significant positive trials required for FDA approval, regardless of the lack of benefit or even harmful effects in the majority of their trials. This means that, astonishingly, two positive trials are all that is required for a drug to be approved, regardless of the many trials that may have previously failed.
To make matters worse, long-term safety studies are not required during the FDA approval process, and pharmaceutical companies are not obligated to publish all data from their trials, including negative results. As a result, pharmaceutical companies can selectively publish positive results and withhold negative data, creating a distorted image of the drug's efficacy and safety. This selective reporting contributes to physicians believing in the effectiveness of these drugs, based solely on the published data, despite the fact that the published data reveals only marginal improvements compared to placebo without any clear real world clinical relevance.
What About the People Who Report Benefit?
Antidepressant drugs have gained an unwarranted reputation that exceeds the actual evidence. It's important to remember that the idea of depression or anxiety as an illness requiring medical treatment is a relatively new development. In fact, many of the emotional difficulties that people experience are episodic and often resolve on their own, without the need for formal intervention. This has been the case throughout human history, and without a historical perspective, we may wrongly attribute the improvement to the use of modern medications.
The power of the placebo response should not be underestimated. Placebo effects are highly dependent upon the perceptions and expectations of the individual receiving treatment, and can be greatly influenced by various factors. For instance, placebo effects may be enhanced or reduced depending on the factors that affect the individual's expectations. When patients are given psychoactive substances that affect the brain, they are often aware that they are receiving medication, which can further amplify their expectations of improvement. Furthermore, individuals with depression may be susceptible to biases that foster a sense of hopelessness and despair, leading them to believe that they are inherently flawed or that their situation is hopeless. The notion that a drug could provide a quick solution to this emotional pain and repair what is damaged may further enhance the placebo response.
Antidepressant drugs are widely available and frequently prescribed for individuals reporting mood or anxiety concerns. While short-term benefits MAY be associated with their use for a small percentage of people, there is no credible evidence to support their long-term use beyond 3-4 months. It is crucial to acknowledge that there are potential risks and debilitating side effects that must be carefully considered against the potential benefits.
How Do These Drugs Affect People?
In 2022, a systematic review in the journal Molecular Psychiatry reviewed the evidence from all the main areas of research examining the link between serotonin and depression. The authors found that none of these areas of research showed convincing evidence that depression is caused by low serotonin. In fact, there was little evidence of any abnormality of serotonin in people with depression.
It is important to acknowledge that while selective serotonin reuptake inhibitors (SSRIs) do modify serotonin levels in the brain, there is no evidence to support the theory that they are correcting an underlying deficiency. Therefore, we must consider that they are actually altering our normal brain chemistry. The effects of drugs that change brain chemistry can impact our mental states and emotions in ways similar to alcohol, which is known to temporarily alter mood. Antidepressants do not have the same chemical or behavioral effects as alcohol, but they have been reported to numb emotions in a general sense for some people, which could be interpreted as a short-term benefit.
Dr. Joanna Moncrieff, a prominent psychiatrist from the UK, has proposed an alternative model of understanding the effects of antidepressants, which she calls the "drug-centered" model. This model emphasizes the ways in which antidepressants alter the body and the brain, and how they can affect feelings and behavior.
According to this model, antidepressants do not simply correct a chemical imbalance, but rather exert a broader influence on brain chemistry and functioning. They can modify the way in which neurotransmitters like serotonin are produced, released, and reabsorbed, and this can in turn impact a range of mental and emotional states. These drugs impact the entire body. What does this mean? Instead of correcting a chemical imbalance these drugs actually INDUCE a chemical imbalance and perturb normal brain functioning that can have serious health effects.
With that being said, it is important to acknowledge that there might be certain short-term advantages to taking an antidepressant drug, some of which may not necessarily arise from the inherent properties of the drug. Nonetheless, what SHOULD a risk/benefit analysis include when discussing antidepressant drugs?
Potential Benefits
1. Hope & Optimism. Many individuals grappling with depression and anxiety harbor a deep sense of hopelessness, believing that their suffering is perpetual and that they are inherently flawed. In our contemporary society, which portrays mental health as a biological illness, there is an amplified sense of optimism that medical interventions can alleviate their distress and address the underlying issue. This sense of hope can be a powerful motivator, inspiring cognitive and behavioral changes that are essential in overcoming depression.
2. Enhanced Placebo Response. An enhanced placebo response refers to a phenomenon where the placebo effect is more pronounced or stronger than expected. The placebo effect is the psychological and physiological response that occurs when a person experiences improvements in symptoms or conditions after receiving a treatment or intervention that is inactive or has no therapeutic effect. In the case of an enhanced placebo response, individuals may experience more significant improvements in their symptoms or conditions simply due to their beliefs, expectations, or perceptions about the treatment they are receiving, even if the treatment itself does not have any active ingredients or therapeutic properties. Antidepressants are a psychoactive substance that acts on the brain so when studied in trials the participants and treatment providers often knew they received the drug.
The enhanced placebo response can be influenced by a range of factors, including the individual's level of trust and confidence in the treatment, the context and presentation of the treatment, and the overall therapeutic relationship with the healthcare provider. In addition to these factors, language itself can have a powerful impact. The way we label a medication, such as referring to an SSRI as an "antidepressant," can significantly shape our expectations and beliefs about its effectiveness. The use of such terminology can influence our perception of the medication's purpose and potential benefits, potentially amplifying the placebo response. It highlights the important role that language plays in shaping our psychological and physiological responses to treatments, emphasizing the need for careful consideration of the words we use when discussing medications and their effects.
3. Validation of Emotional Pain. Individuals grappling with depression and anxiety often experience invalidation, both from themselves and others. They may be self-critical and feel shame about their struggles. Framing depression or anxiety as a medical condition can provide acknowledgment and validation, affirming the legitimacy of their experiences.
By recognizing these conditions as medical issues, individuals can alleviate the burden of self-blame and shame that may accompany their struggles. They may find solace in understanding that their difficulties stem from an illness rather than personal shortcomings. This acknowledgment of pain validates their internal experience and assures them that their feelings are valid and deserving of attention. It can lead to improvements in their well-being and alter how others react to them, fostering more understanding and supportive relationships.
4. Emotional Numbing. An initial therapeutic benefit of antidepressant medication can manifest as a form of anxiolysis, which is experienced by many individuals as a reduction in anxiety or emotional numbing. This emotional numbing, particularly for those in a state of high emotional distress, can be perceived as relieving and instill hope for future recovery. However, it is important to recognize that this perceived benefit of emotional numbing may not be universally experienced and can have adverse effects on some individuals.
While some may find emotional numbing to be a temporary relief, others may report feeling emotionally distant or detached, resulting in a diminished range of positive emotions. Furthermore, sexual side effects, such as a numbing sensation in the genitals, can also occur. It is crucial to differentiate this numbing effect from a genuine recovery from a depressive episode, as emotional numbing is inconsistent with current scientific understanding of emotion regulation and the process of recovering from emotional disorders.
It is important to note that this effect is often temporary. Over time, the body tends to adapt to the drug, which may result in a decrease in its effectiveness. To maintain the desired therapeutic outcome, one might require an increased dosage of the medication or the addition of other drugs. The process of adaptation in the body can lead to a tolerance to the medication's effects. This means that the initial anxiolytic benefits experienced may diminish over time.
Potential Risks
The potential side effects of antidepressant drugs can be extensive and can vary significantly from person to person. It is crucial to thoroughly review the drug's official website or accompanying materials to understand the specific potential side effects associated with that particular medication.
However, it is important to note that physicians have been known to downplay the severity and prevalence of adverse drug reactions. They may rely on information provided by drug sales representatives or attend industry-sponsored continuing education seminars, which can sometimes present a biased perspective.
For the purpose of this document, I will link to published research that highlights the most severe and debilitating adverse drug reactions associated with antidepressant use.
Worsening Symptoms, Suicide, Self-Injury & Violence
Numerous sources of evidence have consistently demonstrated that selective serotonin reuptake inhibitors (SSRIs) commonly contribute to or exacerbate a wide range of abnormal mental and behavioral conditions.. Antidepressants may double the risk of suicide and SSRI’s were the most strongly and consistently implicated drug associated with violence in a review of prescription drugs. Inexplicably, despite research indicating an increased risk of suicidal thoughts and events compared to placebo, these drugs continue to be commonly recommended as first-line treatments for individuals experiencing suicidal ideation. Additionally, it appears that a percentage of people exhibit alterations in how they metabolize these drugs, increasing several overlapping clinical phenomena associated with violence against self or others that include:
Stimulant profile: SSRIs can induce a spectrum of stimulant-like effects, varying from mild agitation to manic psychoses. These manifestations can significantly impact an individual's mental state and lead to a range of abnormal behaviors.
Agitated depression: Some individuals may experience an increase in agitation and restlessness when taking SSRIs, which can intensify depressive symptoms and contribute to a worsening mental condition.
Obsessive preoccupations: SSRIs have been associated with the development of obsessive thoughts or preoccupations that are uncharacteristic or unfamiliar to the individual. These intrusive and distressing thoughts can significantly impact mental well-being.
Akathisia: Akathisia refers to a state of inner restlessness and an inability to remain still. It can manifest as an adverse reaction to SSRIs, leading to extreme discomfort and an escalation of abnormal behavior, including violent acts against self or others.
These adverse reactions have been consistently reported in clinical accounts, controlled clinical trials, and epidemiological studies conducted in both children and adults. Recognizing these potential adverse drug reactions is crucial in preventing misdiagnosis and the exacerbation of iatrogenic (drug-induced) disorders. Abrupt withdrawal from the offending drugs, adding additional drugs and increasing the dosage of the drug when experiencing adverse drug reactions can increase the risk of a life threatening event.
Post SSRI Sexual Dysfunction (PSSD)
Sexual dysfunctions are well-known side effects of selective serotonin reuptake inhibitor(SSRI) use. Altered libido; erectile dysfunction; vaginal dryness; ejaculatory disorders; pleasureless, weak or “muted” organisms; and loss of penile/clitoral size are frequently reported by patients treated with SSRIs. Moreover, these antidepressant-emergent sexual dysfunctions do not always resolve after discontinuation of the medication and can persist indefinitely. I recommend a thorough review of the PSSD network website to understand this debilitating side effect. Most people I have spoken to who suffer from PSSD were not advised of this risk and would never have taken the drug if adequately informed.
Emotional Blunting and Anhedonia
As its name implies, emotional blunting refers to a state of numbing where both positive and negative emotions become muted. Emotional blunting is reported by nearly half of depressed patients on antidepressants. These patients report a noticeable decrease or limitation in the intensity and frequency of emotions that are essential for everyday functioning. This may manifest as difficulties in expressing emotions through crying, challenges in empathizing with others' emotions, or a loss of enjoyment in activities that used to bring pleasure (known as anhedonia).
In some cases this could exacerbate hopelessness and increase suicide risk. As mentioned earlier, in the short term this could be interpreted as a “positive response” for some who are experiencing intense emotional distress, but continued inability to experience emotions should not be considered a recovery from depression.
Weight Gain or Risk of Metabolic Dysfunction
Metabolic syndrome (MetS) encompasses a collection of health conditions including obesity, insulin resistance, hypertension, impaired glucose tolerance or diabetes, hyperinsulinemia, elevated triglycerides, and low high-density lipoprotein (HDL) concentrations. Commonly prescribed antidepressant medications have been associated with weight gain, as supported by as a study revealing that individuals using these medications for an extended duration were 21 percent more likely to experience weight gain.
After two years of antidepressant use, individuals who were previously categorized as having a "normal" weight had a 29 percent higher likelihood of becoming obese or overweight. There is mounting evidence that SSRI’s could be associated with an increased risk of developing metabolic syndrome. Furthermore, it is important to acknowledge that in the acute phase of Prozac usage, some individuals may encounter decreased appetite and experience weight loss as a potential side effect.
Sleep Disturbance
Psychiatric medications are commonly associated with sleep disturbances. This runs the gamut from affecting dreams, increasing sleep time, encouraging sleep or creating insomnia. Insomnia is a common side effect listed on SSRI drug websites and should be considered. There exists a varying level of evidence linking the use of antidepressant medication to the parasomnias. Parasomnias are a diagnostic grouping of sleep disorders that include abnormal sleep-related behaviors, dreams, or autonomic changes that manifest during transitions from wakefulness to sleep, or within transitions between sleep stages. SSRIs are known to profoundly suppress the rapid-eye-movement (REM) stage of sleep, which is where dreams occur. This can lead to daytime fatigue.
Withdrawal Syndrome
Extensive research has revealed the alarming frequency of antidepressant withdrawal reactions, with incidence rates ranging from 27% to as high as 86% (weighted average of 56%). These symptoms can be distressingly severe and incapacitating, causing significant challenges for those affected.
It is crucial to understand that experiencing withdrawal does not necessarily indicate a relapse of the underlying condition requiring further medication, contrary to what some medical professionals have suggested to their patients. Abruptly stopping an antidepressant can be life threatening. Severe withdrawal reactions have included psychosis, akathisia, severe insomnia, suicidal ideations, homicidal ideations, aggression, and severe sleep disturbance.
Notably, discontinuation symptoms have been observed not only after abrupt discontinuation but also after gradual tapering of antidepressants. Moreover, the prevalence of these symptoms varies depending on the pharmacological profile of the specific antidepressant being used. To gain a comprehensive understanding of the existing literature on this topic, we invite you to explore a thorough review by clicking here
“Altering serotonin levels may have unintended consequences, There are a lot of biochemical mechanisms in the body to keep our neurotransmitters stable. Taking an SSRI perturbs that system. Withdrawal symptoms might actually be the result of the body struggling to recover its natural serotonin balance, desperately trying to get things back to normal.”
-Jay Amsterdam, MD
Psychopharmacologist & Emeritus Professor of Psychiatry
University of Pennsylvania
Pregnancy Risks
A new study in Psychological Medicine finds that babies born to mothers taking antidepressants were more than six times as likely to have neonatal withdrawal syndrome—including:
breathing problems
irritability/agitation, tremors
feeding problems
Seizures—than those born to mothers taking other types of drugs.
More than 80% of the reported symptoms were classified as serious.
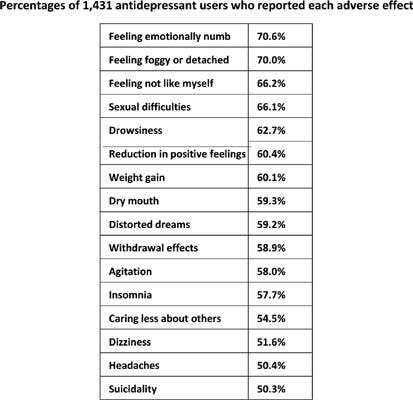
How Prevalent are These Adverse Effects?
An online survey gathered responses from 1,431 adult antidepressant users across 38 countries. The results were striking: 61% of participants reported experiencing at least ten out of the 20 specified adverse effects. The most common effects included emotional numbness (71%), feeling foggy or detached (70%), not feeling like oneself (66%), sexual difficulties (66%), drowsiness (63%), and a reduction in positive feelings (60%). Shockingly, 50% reported experiencing suicidality as a result of the medication.
Withdrawal effects were reported by 59% of respondents, and 40% expressed concerns about addiction. Rates of adverse effects were higher among individuals prescribed multiple antidepressants or those concurrently taking antipsychotics. Younger age and longer use of antidepressants were positively associated with a higher number of adverse effects. Disturbingly, one third of participants did not recall being informed about any side effects by their prescribers. Even more concerning, less than 5% were informed about the risks of suicidality, emotional numbing, withdrawal effects, or addiction.
Conclusions
When evaluating the risks and benefits of antidepressant drugs, it becomes increasingly difficult to present a convincing argument that the benefits outweigh the risks. In my understanding, if individuals were adequately informed about the comprehensive extent of risks associated with these medications, the majority would likely reach a similar conclusion and opt against treatment. Moreover, it is crucial to acknowledge that the perceived benefits of antidepressants are frequently influenced by the potent placebo response, a significant factor in medical treatments.
In a forthcoming article, I will explore the wide range of alternative options available to individuals facing challenges with depression and anxiety.
P.S. A heartfelt thank you to all my subscribers! While I offer all my articles for free, your support through donations would be greatly appreciated. Your generosity helps me continue delivering valuable content. Thank you for being a part of this incredible community! If you are interested in more thought provoking conversations please subscribe and download the Radically Genuine Podcast with Dr. Roger McFillin.



I’m so glad you’re speaking up about this. I’ve never been on an antidepressant. But, my OB/GYN prescribed me one after a devastating pregnancy loss. I actually questioned him and said that it was normal to feel this way after losing a child. He said, “well you have it just in case.” In case of what? I threw it away. It occurred to me then that many, many doctors are prescribing these mind altering drugs “just in case” instead of having any compassion for grief, sadness and anxiety... all perfectly normal human emotions. It was almost as if he couldn’t deal with my grief, so he prescribed medication. I dealt with my grief, leaning on my Faith. Something else this society has degraded to it’s own detriment.
I came across this type of research (and your work & podcast interviews) while working on a documentary linking health & infrastructure. I knew it'd get people fired up to say walking & bicycling might be your best treatment for anxiety & depression, but wow did I underestimate the REEEEEs online.
Thanks for being on Substack!