The STAR*D Fraud: How Psychiatry's Landmark Study Deceived the World
The scientific misconduct that continues to justify antidepressants
Despite overwhelming evidence of fraud and misconduct in antidepressant research, an alarming number of medical professionals continue to operate behind what can only be described as a pharmaceutical iron curtain. Every day, countless patients are prescribed powerful psychotropic drugs based on studies now known to be manipulated and misleading.
The landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, once touted as the gold standard for antidepressant efficacy, has been exposed as a monument to scientific deception. Yet, in a shocking display of institutional inertia and willful ignorance, the original study has not been retracted.
It continues to serve as justification for antidepressants as a front-line intervention, perpetuating a massive fraud on an unsuspecting public. This isn't mere academic oversight; it's a calculated suppression of truth that would have decimated public trust in the medical establishment.
Had the real results been acknowledged, psychiatry's status as a legitimate medical specialty would have crumbled overnight. The product liability cases would have been unprecedented, potentially bankrupting pharmaceutical giants and exposing countless physicians to malpractice claims. This information remains ignored by mainstream corporate media, leaving millions in the dark about the true nature of these widely prescribed medications.
Investigative journalist and author Robert Whitaker has unearthed a scandal of staggering proportions, one that has been systematically ignored by both corporate media and the medical establishment. His exposé, The STAR*D Scandal: Scientific Misconduct on a Grand Scale lays bare a level of fraud that strikes at the very heart of modern psychiatric practice. His article examines H. Edmund Pigott and colleagues' 2010 and 2023 analyses of the STAR*D clinical trial data, which repeatedly exposed protocol violations that inflated results.
This article aims to distill Whitaker's extensive investigation into accessible key points, shining a light on a controversy that has been kept in the shadows for far too long.
History Lesson
In the years leading up to 2001, the landscape of antidepressant research was dominated by short-term, industry-sponsored clinical trials. These studies, often lasting just 6-8 weeks, were typically designed and funded by pharmaceutical companies with a vested interest in showcasing their drugs in the best possible light.
Such trials frequently employed strict inclusion criteria, selecting patients most likely to respond positively while excluding those with complex medical histories or comorbid conditions. This approach, while methodologically sound for regulatory approval, left a significant gap in understanding how these drugs performed in real-world clinical settings over extended periods.
The pharmaceutical industry has long been criticized for employing various tactics to enhance the apparent efficacy of their antidepressant drugs in clinical trials. These strategies significantly skew results in favor of the medication being tested.
One common practice is "placebo washing," where all potential participants are given a placebo for a brief period before the actual trial begins. Those who respond positively to the placebo are then excluded from the study, effectively weeding out individuals who might dilute the apparent effectiveness of the drug during the trial.
A particularly alarming practice in some antidepressant trials involves the handling of patients who develop suicidal thoughts or behaviors while on the study drug. Rather than recording these as potential serious adverse effects of the medication, early trials have been known to remove these patients from the study entirely or reclassify their symptoms under more benign-sounding terms like "worsening depression" or "emotional lability."
This practice serves a dual purpose for pharmaceutical companies: it eliminates data points that could raise safety concerns about their drug, while simultaneously removing participants who are not responding well to the treatment. The result is a dataset that appears to show a lower incidence of suicidality and a higher efficacy rate than may actually be the case. This manipulation of data not only misrepresents the true risk-benefit profile of the drug but also endangers future patients by understating the risk of suicidal ideation as a side effect.
Perhaps most concerning is the practice of abruptly discontinuing participants in the placebo arm from their current psychiatric drugs at the start of the trial. This sudden cessation can induce severe withdrawal symptoms that mimic or exacerbate depression, making the placebo group appear to fare worse in comparison to those receiving the new drug. These withdrawal symptoms are often misinterpreted as a worsening of the underlying depression, further inflating the apparent efficacy of the drug being studied.
Collectively, these methods create a trial environment that's far removed from real-world clinical scenarios, leading to an overestimation of a drug's effectiveness when prescribed to the general population.
This short-term industry funded trials left crucial questions unanswered: do people taking antidepressants actually get better or worse over time? This concept, known as the "stay well rate," is vital for understanding the true efficacy of these drugs in managing depression as a chronic condition. Without long-term data, it's impossible to determine whether initial improvements are maintained, whether patients relapse despite ongoing treatment, or whether some even experience a worsening of their condition over time.
Recognizing these concerns, the National Institute of Mental Health (NIMH) initiated the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study in 2001. STAR*D was conceived as a response to the need for long-term, unbiased research that would reflect the true effectiveness of antidepressants in diverse patient populations encountered in everyday clinical practice.
STAR*D TRIAL
The STAR*D study was a landmark research project launched in 2001 by the National Institute of Mental Health (NIMH). Running until 2006, it was designed to be the largest and longest study ever conducted to evaluate depression treatment in the United States. The primary goal of STAR*D was to assess the effectiveness of different treatment strategies for patients with major depressive disorder in real-world clinical settings over this five-year period.
What made STAR*D unique was its focus on "real-world" patients during this time frame. Unlike many clinical trials that exclude patients with complex medical histories or comorbid conditions, STAR*D aimed to include a diverse range of patients who better represented those typically seen in everyday clinical practice between 2001 and 2006.
The study involved 4,041 participants from various clinical settings across the country, including primary care and psychiatric clinics. STAR*D was structured in multiple treatment steps, allowing patients who didn't respond to initial treatments to try different medications or combinations, mirroring how depression was often treated in actual clinical practice during the early 2000s.
Structure of the study:
Step 1: All participants started with citalopram (Celexa), an SSRI antidepressant. This phase lasted up to 14 weeks.
Steps 2-4: Participants who didn't achieve remission in a previous step were offered different treatment options:
Switch to a different antidepressant
Add another medication to their current treatment
Add cognitive therapy
Try other combinations of medications
Each step lasted up to 12 weeks.
Key points about the study design:
1. Real-world approach: Unlike many clinical trials, STAR*D aimed to include a diverse range of patients, including those with other medical conditions.
2. Flexible dosing: Medications could be adjusted based on patient response and side effects.
3. Patient choice: In later steps, patients could choose from available treatment options.
4. Multiple outcome measures: The study used various scales to assess depression, including the Hamilton Rating Scale for Depression (HRSD) and the Quick Inventory of Depressive Symptomatology (QIDS).
5. Long-term follow-up: Patients who achieved remission were followed for up to 12 months to assess long-term outcomes.
The study was designed to mimic real-world treatment scenarios and provide insights into the effectiveness of different treatment strategies for depression that doesn't respond to initial interventions.
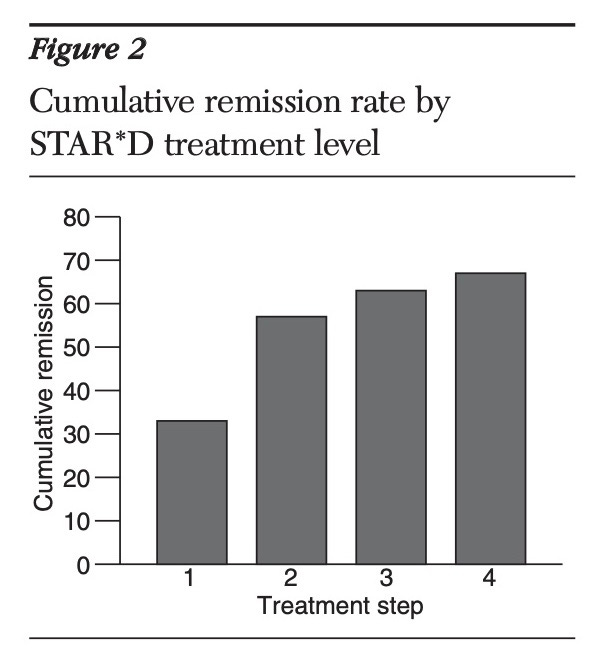
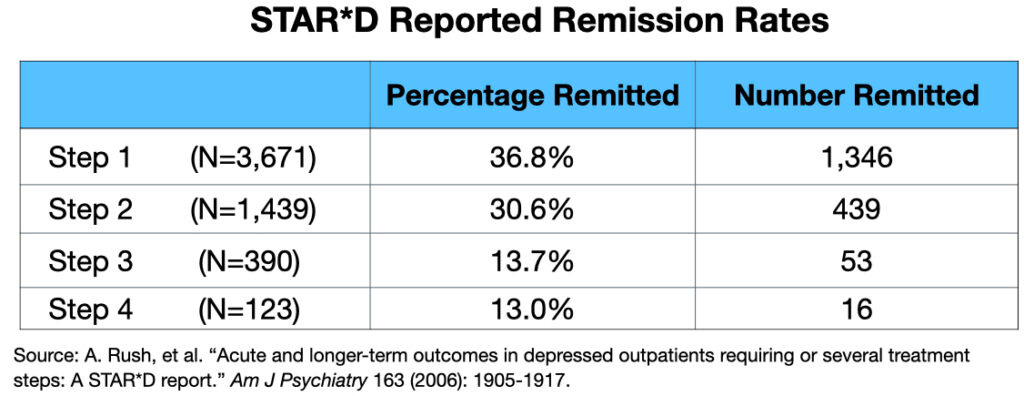
The original summary paper, published in November 2006 in the American Journal of Psychiatry, prominently reported a cumulative remission rate of 67% after four treatment steps.
This figure became widely cited and promoted in both academic and public discussions about the study. However, the actual long-term outcomes were not clearly presented. The paper devoted only two short paragraphs to the one-year follow-up results, burying crucial information about sustained wellness in a complex, poorly explained graphic.
Source: Gaynes, et al. “What Did STAR*D Teach Us? Results from a Large-scale, Practical, Clinical Trial for Patients with Depression.” Psychiatric Services 60 (2009):1439-1445.
Subsequent communications about STARD continued to emphasize positive outcomes while downplaying less favorable long-term results. For instance, in 2009, NIMH director Thomas Insel stated that "at the end of 12 months, with up to four treatment steps, roughly 70% of participants were in remission."
The publication of these results was a significant event in the field of psychiatry, as it was expected to provide definitive guidance on the effectiveness of antidepressant treatments in real-world clinical settings. The STAR*D findings quickly became influential, shaping both clinical practice and public perception of antidepressant efficacy in the years following their publication.
Scientific Misconduct
In 2010, psychologist Ed Pigott and his colleagues published a groundbreaking deconstruction of the STAR*D trial, revealing significant scientific misconduct. You can access this again here: READ GROUNDBREAKING ANALYSIS and 2023 Publication.
Here are the key points:
1. Pigott obtained the original STAR*D protocol through a Freedom of Information Act request.
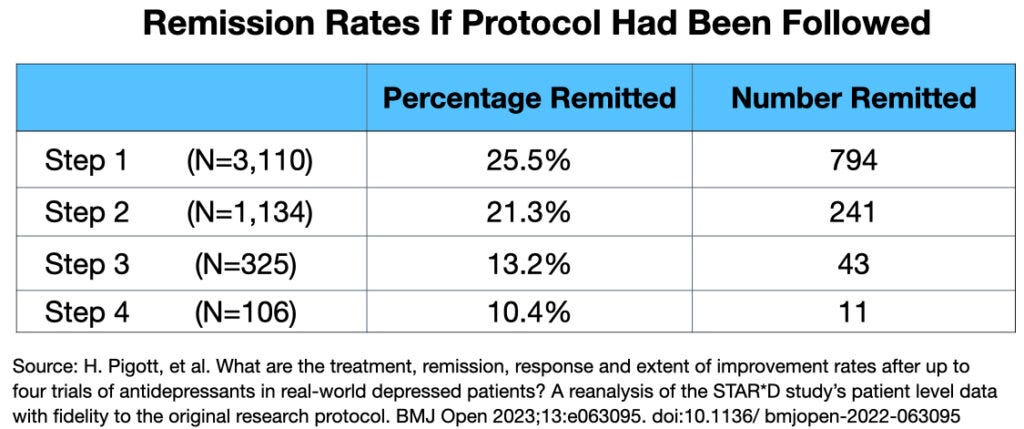
2. By comparing the protocol to the published results, Pigott identified multiple deviations that artificially inflated the reported remission rates.
3. Pigott's analysis showed that if the protocol had been followed, the cumulative remission rate would have been 38%, not the reported 67%.
4. Most strikingly, Pigott discovered that only 3% of the 4,041 patients who entered the trial had actually remitted and stayed well for the full one-year follow-up period.
5. This 3% "stay-well" rate was hidden in a confusing graphic in the original STAR*D publication and not prominently reported.
6. When asked about Pigott's findings, STAR*D investigator Maurizio Fava confirmed that the 3% figure was "reasonable and not incompatible with what we had reported."
7. Despite these revelations, the original STAR*D papers were not retracted, and the investigators faced no censure for their misconduct.
8. The American Psychiatric Association and academic psychiatrists in the United States have remained largely silent on this issue.
This exposé revealed that the most influential antidepressant study of the 21st century was based on manipulated data and hidden negative outcomes, raising serious questions about the efficacy of antidepressants and the integrity of psychiatric research.
Institutional Corruption & Criminal Negligence
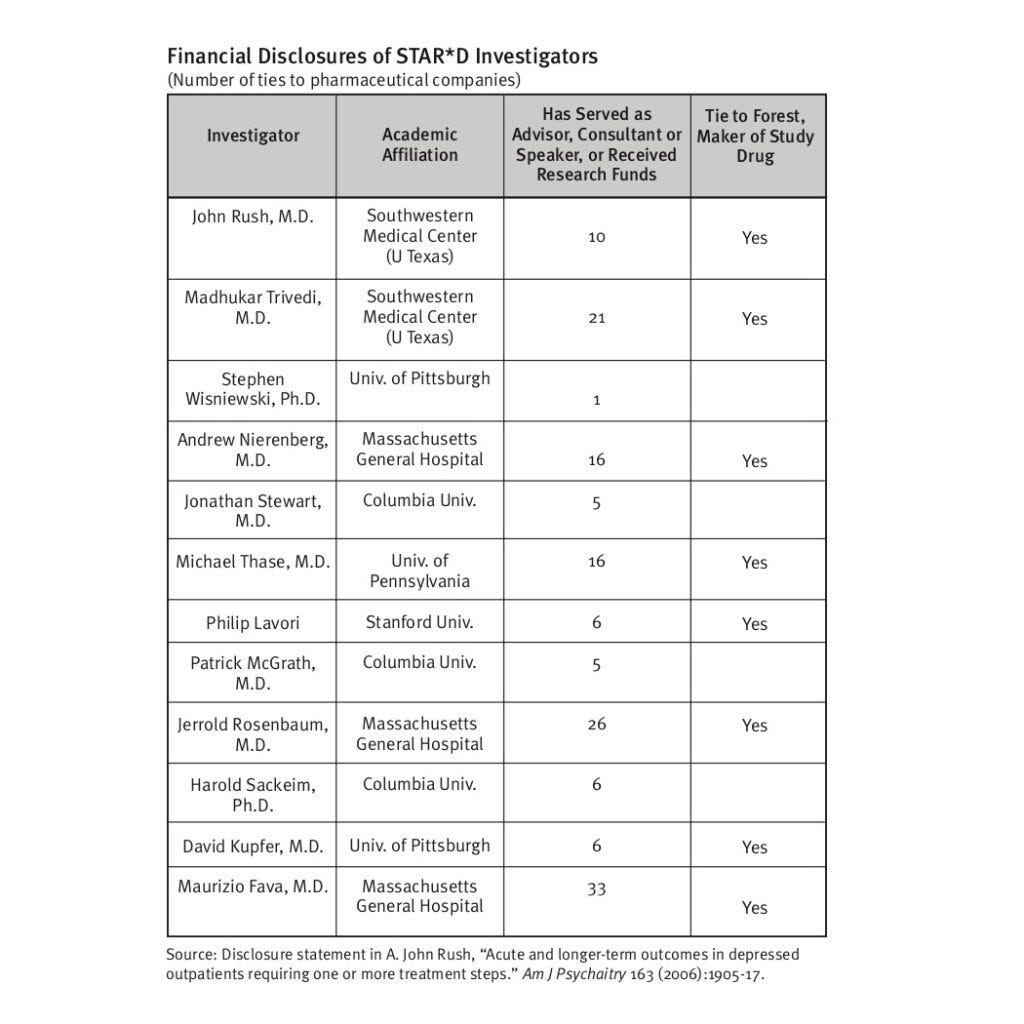
Robert Whitaker and Lisa Cosgrove, in their book "Psychiatry Under the Influence," exposed the STAR*D trial as a prime example of "institutional corruption" in psychiatry. This corruption stemmed from two main sources: psychiatry's guild interests and the STAR*D investigators' extensive financial ties to pharmaceutical companies.
The American Psychiatric Association, functioning more as a trade association promoting its members' interests, has long championed antidepressants as safe and effective (where have we heard that line before?) Accurate reporting of STAR*D results - showing only 35% remission after four treatment steps and a mere 3% staying well after one year - would have severely undermined this narrative and decreased both prescriptions and demand for these drugs.
Instead, through protocol deviations, imagined remissions, and concealment of long-term results, the investigators transformed STAR*D into a false success story. This protected not only their "product" but also psychiatry's public image, portraying skilled professionals helping two-thirds of patients become "symptom free."
Despite NIMH funding, pharmaceutical influence persisted due to extensive financial ties between STAR*D investigators and drug manufacturers. The STAR*D scandal exposes a profound betrayal of public trust and scientific integrity. This fraud has misled millions, justifying the mass prescription of dubiously effective drugs. The fabricated 67% remission rate is not just bad science; it's a dangerous deception that has potentially harmed countless patients.
The psychiatric establishment's silence in the face of this exposed fraud is damning. It reveals a profession more committed to self-preservation than patient welfare. By refusing to retract these fraudulent findings, the field of psychiatry is complicit in ongoing harm.
This is not merely an academic dispute. It's a public health crisis that demands immediate action. Every psychiatric guideline, every prescription, must now be questioned. The STAR*D fraud undermines the credibility of psychiatry as a medical specialty and calls for a complete overhaul of mental health research and treatment practices.
Consequences
The STAR*D study's fraudulent findings have become the cornerstone of modern antidepressant prescription practices, perpetuating a cycle of ineffective and potentially harmful treatment.
When a patient first presents with depressive symptoms, doctors, armed with the false promise of a 67% remission rate, confidently prescribe an initial antidepressant. When this fails—as it often does—rather than questioning the efficacy of these drugs, clinicians fall back on STAR*D's deceptive narrative of success through multiple treatment steps. Patients are then subjected to a pharmaceutical merry-go-round, switching between various antidepressants or combining them, all under the misguided belief that persistence will eventually lead to remission.
This approach, directly stemming from STAR*D's manipulated data, traps countless individuals in a prolonged, often futile, and potentially dangerous course of treatment. What's more, antidepressants come with a host of serious adverse reactions and negative health consequences, including sexual dysfunction, weight gain, emotional numbing, and increased risk of suicidal thoughts. Tragically, when patients experience these side effects or a worsening of their condition, it's frequently misinterpreted as a deterioration of their underlying mental illness rather than a consequence of the medication.
This misattribution creates a pernicious cycle: drug-induced symptoms are seen as justification for more aggressive pharmacological intervention, leading to increased doses, additional medications, or different drug combinations. As a result, patients find themselves ensnared in psychiatric care, their initial problems compounded by iatrogenic harm, while the true culprit—the medication itself—remains unquestioned.
The STAR*D fraud has thus not only misinformed initial treatment decisions but has created a self-perpetuating system that keeps patients tethered to ineffective and often harmful psychiatric interventions, all under the guise of evidence-based medicine.
This story needs to be told.
All who read this… share it. Talk about it. Be loud.
Are you a clinician?
You have an ethical obligation to share this information and inform people who have been mislead. The Conscious Clinician Collective is a non-profit trying to align all ethical mental health and healthcare practitioners who believe in informed consent and medical freedom. I am trying to develop a data base and grow a site that provides this important information. Please visit the website through the link below. We need your help!






If psychiatrists aren't acknowledging this study or speaking out about it, shouldn't we be even more concerned about all the GPs who are prescribing so many of these drugs and who are definitely not keeping up with the research on them?
Thanks doc for all your work.
Also, just letting you know that I finally wrote 'the' article about what we experienced here. I have been getting messages from people that they cannot even look at what I wrote, even now in 2024. https://vicparkpetition.substack.com/p/memory-holed-what-we-endured
You will have an absolute field day with the psychological elements here.